Avulux Migraine & Light Sensitivity Lenses
An Overnight Success A Decade In The Making
Born From Cutting Edge Research
Avulux Migraine & Light Sensitivity Lenses officially launched in 2018, and that only happened after a long path of clinical research and development. In 2011, a neuro-ophthalmologist, photonics researcher, and ophthalmic entrepreneur researched and patented a new lens technology, that would later become Avulux, with the goal of precisely filtering harmful light linked to migraine attacks.
After extensive development and testing multiple prototypes, the patented Avulux Migraine & Light Sensitivity Lens formulation was released to the public. Avulux has engineered the only lens that has proven clinical and statistical significance, at the highest scientific standard, in helping people with migraine.
The History Behind Developing the Only Clinically-Proven Lens for People Living with Migraine and Light Sensitivity
2010
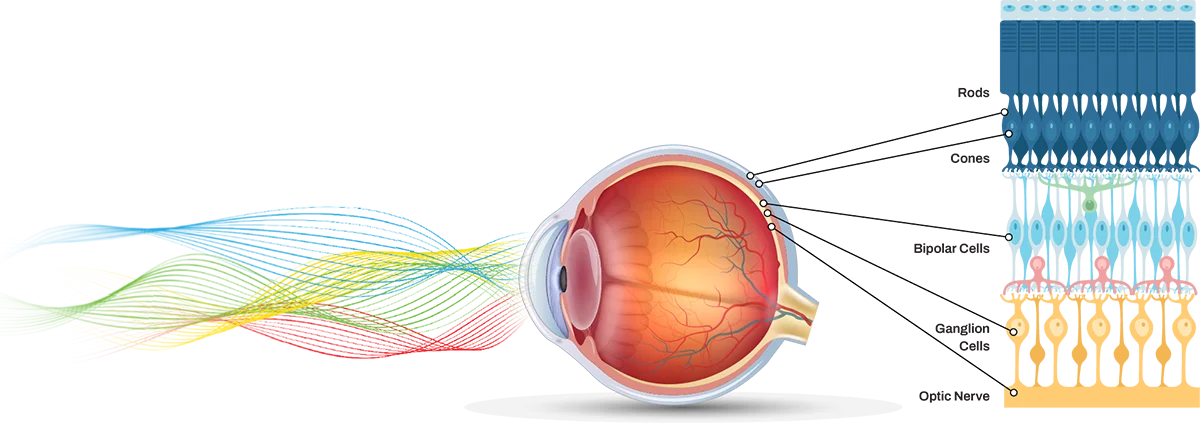
Melanopsin is a photopigment that is released when cells at the back of the eye, called intrinsically photosensitive retinal ganglion cells (ipRGCs), are exposed to light in the upper blue and amber range. Research in 2010 linked melanopsin-secreting ipRGCs to pain in people with migraine.
2011
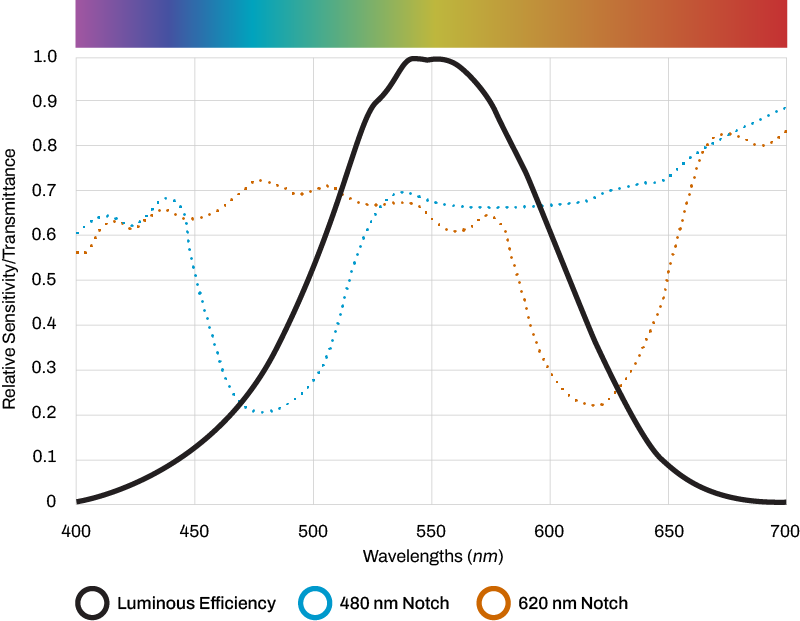
Research & development into a next-generation 480nm precision filter. Lens patent filed by neuro-ophthalmologist and optical engineer at University of Utah.
2015
2017
2016 study published. Began improving lens design with additional filtration properties for improved efficacy, color neutrality, and clarity.
Using nano-molecular technology, current Avulux lens created.

2019
2020